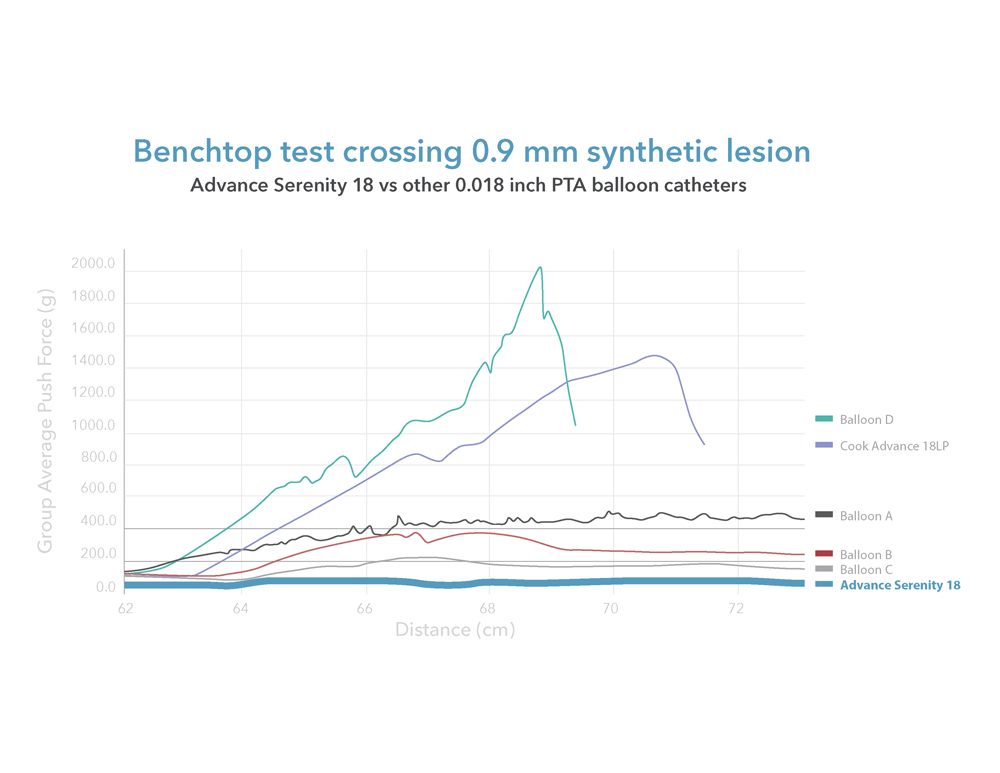
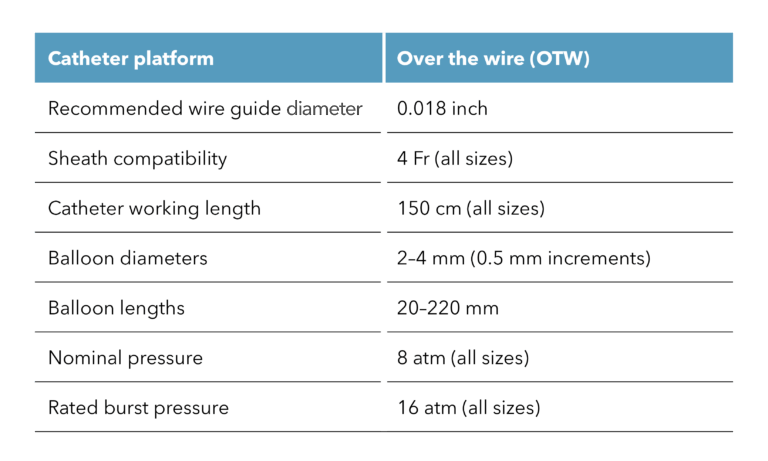
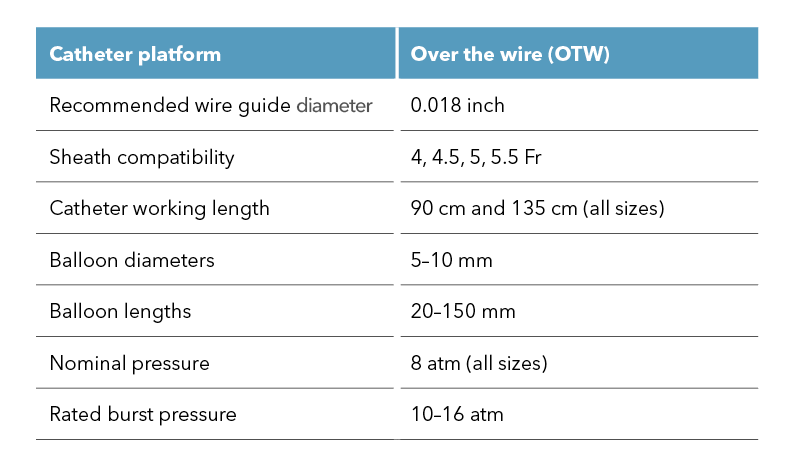
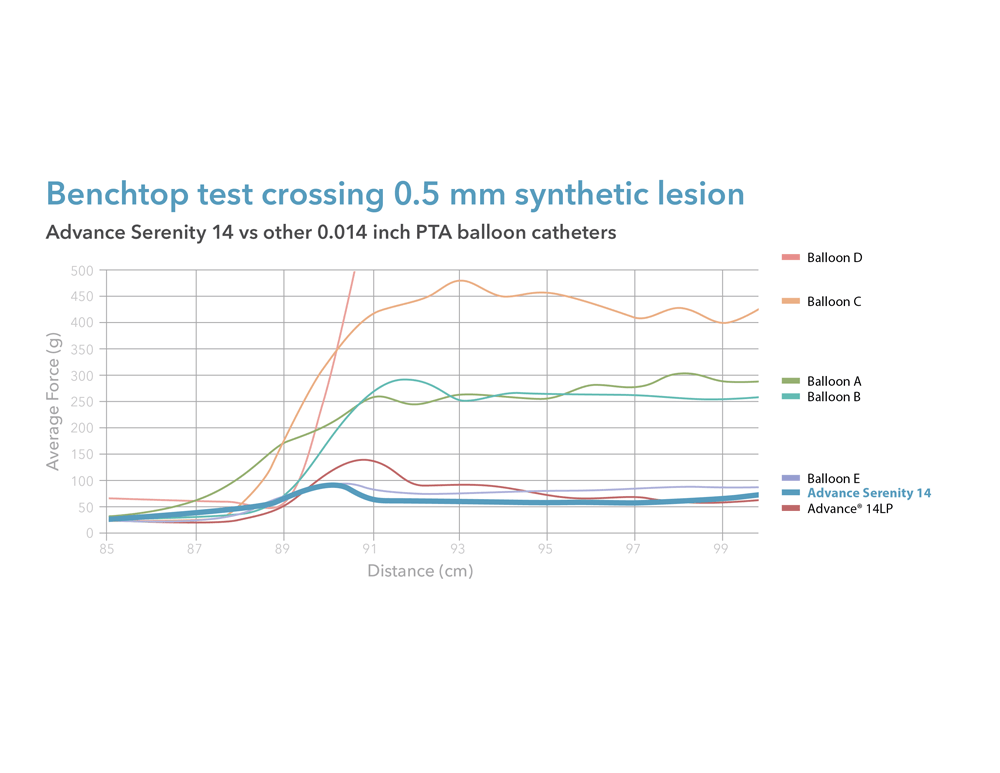
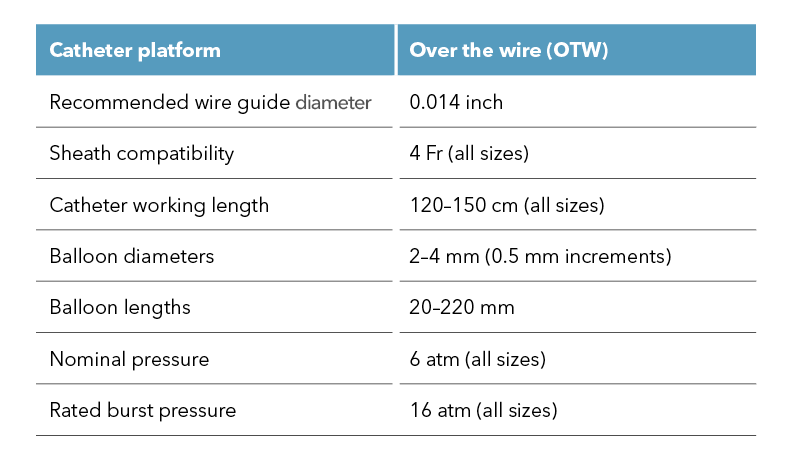
Are you treating peripheral artery disease (PAD) in the infrapopliteal or femoropopliteal regions? We are excited to introduce our Advance Serenity 0.018 inch and Advance Serenity 0.014 inch balloon dilatation catheters. Whether targeting the infrapopliteal or femoropopliteal arteries, this next-generation technology provides a smoother crossing of tight lesions.