Cook Medical has been listed as one of LinkedIn’s Top Companies in Ireland in 2025, recognising the 25 best workplaces to grow your career in Ireland.
LinkedIn evaluates several key factors when compiling the list, including looking at how companies perform in areas such as employee advancement opportunities, skills development and the overall supportiveness of the company’s culture.
Cook Medical is committed to fostering an inclusive and supportive environment that allows everyone to reach their full potential within Cook.
Carla DiBenedetto, director of HR, commented on this achievement, “Our people are our greatest asset and are at the heart of everything we do. We provide resources and benefits to help our employees achieve their personal and professional goals while actively working to remove any barriers that stand in their way. Therefore, being recognised on this list is a privilege.”
For more information, read the LinkedIn Top Companies 2025, The 25 best workplaces to grow your career in Ireland.

Cook Medical has announced a €3 million investment in renewable and energy-saving technologies.
The project consists of a new 1 megawatt ground-mounted solar PV array on the grounds of the Limerick site, along with 1.2 megawatts of heat pumps to replace the existing chillers, an upgrade of electronically commutated fans, and a new energy management system.

The investment is part of the company’s carbon reduction goals, with the new technologies helping to increase the operational efficiency of the facility in Castletroy and eventually offsetting approximately 50% of carbon emissions.
This project is expected to offset up to 269 tonnes of carbon annually, and over its 25-year life cycle it will remove a total of 6,725 tonnes of carbon. Coupled with the procurement of additional green energy technologies, this will result in the removal of 60% of the carbon from the Cook Ireland site.
This project is supported by the Irish Government through IDA Ireland. Minister for Enterprise Tourism & Employment Peter Burke said, “For almost 30 years, Cook Medical has been present in Ireland and today’s announcement of a €3 million investment in renewable and energy-saving technologies is fantastic news. The combined plans are expected to offset 50% of their carbon emissions, which is in line with the Climate Action Plan to halve Ireland’s emissions by 2030. It is really encouraging to see initiatives being taken by companies focused on carbon reduction measures, sustainability and protecting the environment for future generations. I wish Cook Medical success with these plans and all their future endeavours.”
Commenting on the project, Bill Doherty, executive vice president and managing director of Cook Medical Europe, said, ‘’We are committed to making sustainable choices across our business and reducing our impact on the environment. By integrating solar panels into our operations, we’re able to enhance our efficiency while also reducing our carbon footprint.”
The company is committed to enhancing and maintaining the biodiversity of its site and will continue to safeguard the environment in its plans. In addition to reseeding native wildflowers in all areas under construction, the company will also preserve walking tracks and spaces for wildlife.
CEO of IDA Ireland Michael Lohan said: “This proposed project will position Cook’s Irish operations as an exemplar with its global network, and as a key location of subject matter expertise around energy management systems. Strongly aligned to the National Climate Action Plan’s Enterprise Pillar and IDA Ireland’s sustainability targets, we wish Cook Medical every success with this initiative which has far reaching potential.’’
Upon completion of this project, Cook Ireland will reduce its annual import of electricity by 19% and release that capacity back to the grid. A 70% reduction in the annual use of natural gas consumption is expected from the installation of the heat pumps.
A planning application has been submitted to Limerick City and County Council. If successful, construction on this project is expected to begin in due course.
Bloomington, Ind. — Medical’s Zilver PTX drug-eluting stent (DEShas lower rates of in-stent occlusions among patients with restenosis at three years than Boston Scientific’s Eluvia DES, according to real-world data from the REALDES study. The data, published by Tsuyoshi Shibata, M.D., Ph.D., et al., in the European Journal of Vascular & Endovascular Surgery (EJVES) are the first to compare Zilver PTX and Eluvia in real-world practice at three years.1 These data stand as another proof point of Cook’s relentlessly innovative products.
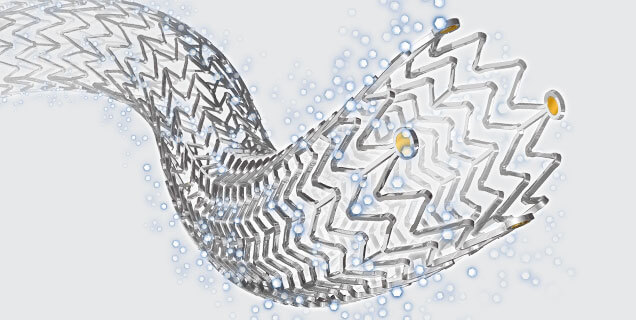
Zilver® PTX®, Cook Medical’s paclitaxel-coated stent
“There have been many unanswered questions on the reliability of the current data comparing the different drug-eluting stents on the market. REALDES helps paint a more complete picture on DES differences, evaluated by independent investigators in a real-world setting,” said Alec Cerchiari, director of product management for Cook’s PAD & Venous specialty. “We are grateful to the physicians and hospitals that participated in this study.”
REALDES is an investigator-initiated, multi-centre, prospective, observational study designed to compare Cook’s Zilver PTX DES and Boston Scientific’s Eluvia DES in a real-world setting for treating symptomatic femoropopliteal lesions. Overall, 200 limbs with native femoropopliteal artery disease were treated with Zilver PTX (96 limbs) or Eluvia (104 limbs) at eight Japanese hospitals between February 2019 and September 2020. The primary outcome measure of this study was primary patency at three years, defined as freedom from restenosis* or occlusion without reintervention.
Key three-year study outcomes include:
- There was no significant difference in primary patency (Zilver PTX, 70.0% vs. Eluvia, 65.2%; P = 0.74) or freedom from CD-TLR (79.4% vs. 76.3%; P = 0.27), despite the Zilver PTX arm having longer lesions (185.7 ± 92 mm vs. 160.0 ± 99 mm; P < .005).
- In patients with restenosis at three years, there was a significantly higher rate of in-stent occlusions (Tosaka class III2) for those treated with Eluvia (57.7%) compared to Zilver PTX (29.2%; P = 0.041).
“These findings contribute valuable insights into optimising treatment outcomes and guiding future stent selection,” said lead author Dr. Shibata of Sapporo Medical University.
To learn more about Zilver PTX, visit Zilver PTX | Cook Medical.
About Cook Medical
At Cook Medical, we are passionate about making unique, quality medical devices and connecting with people to improve lives. Founded on inventing, manufacturing, and delivering medical devices, we provide healthcare professionals with the tools they need to help their patients return to living.
Our commitment to innovation involves bringing new products to market and keeping existing products relevant to a changing healthcare landscape. We believe in using our business to help people and communities thrive by creating inclusive, supportive, and healthy environments.
We are proud of our history of innovative firsts and the impact we have on patients and communities. With headquarters in Bloomington, Indiana, and manufacturing facilities and offices in various global locations, we challenge ourselves to maintain a global perspective while focusing on local impact.
Follow Cook Medical at CookMedical.eu and on LinkedIn.
*Restenosis was characterised as peak systolic velocity ratio > 2.4 based on the duplex ultrasonography findings or the recurrence of ≥50% diameter stenosis as determined by angiographic findings.
1 Shibata T, Iba Y, Shingaki M, et al. Comparative analysis of three-year results of two paclitaxel related stents for the management of femoropopliteal disease in a real-world setting. Eur J Vasc Endovasc Surg.2025; doi: 10.1016/j.ejvs.2025.03.010
2 Tosaka A, Soga Y, Iida O, et al. Classification and clinical impact of restenosis after femoropopliteal stenting. J Am Coll Cardiol. 2012;59:16-23.
Our mission stays the same, but at Cook, we’re relentlessly inventive and proactively adapting to the needs of physicians and patients.
We previously shared three ways Cook is changing. Since then, we’ve made other significant advancements. Here are four more ways Cook is changing to better serve our customers.
1. Growing our product portfolio to fit our future
We’ve recently adjusted our product portfolio to better offer products that align with our vision and serve unmet customer needs with a unique portfolio of products. These portfolio changes include:
Investing in Zenflow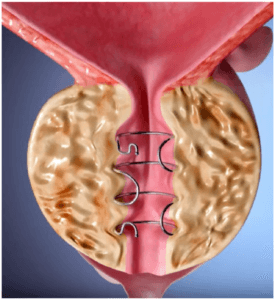
Cook Medical announced a strategic investment in the urology space. Zenflow is a medical device company developing a minimally invasive treatment for urinary obstruction caused by enlarged prostate, or benign prostatic hyperplasia (BPH).“This investment reflects our confidence in the future of Zenflow and the Spring® System technology. Zenflow is aligned with our core focus of developing minimally invasive technologies that restore flow,” said DJ Sirota, senior vice president of Cook Medical’s MedSurg division. “This type of agreement is yet another proof-point to how Cook is changing to focus on our future as product innovators.”
Investing in PillSense
Cook Medical signed an agreement to distribute EnteraSense’s novel blood-sensing capsule, PillSense. We also invested in EnteraSense’s Series B round of funding to support the production and distribution of PillSense capsules. PillSense is a strategic addition to Cook’s portfolio of endoscopic bleed management products, such as Hemospray and the Instinct Plus™ Endoscopic Clipping Device.
Divesting Lead Management products
Cook Medical sold our portfolio of lead management products to Merit Medical. Merit has an existing footprint in the electrophysiology (EP) space and plans to expand their presence in Lead Management. Merit is making significant investment in EP and shows they are committed to growing the business in ways that Cook has not been able to in recent years. This acquisition represents a tremendous opportunity for employees, products and patients while aligning with Cook’s long-term goals.
Divesting the Reproductive Health portfolio
Cook Medical sold our portfolio of Reproductive Health and Assisted Reproductive Technology (ART) products to Astorg. Astorg, an investment firm with deep experience in MedTech across both manufacturing and product businesses with a focus on growth and innovation, acquired and simultaneously combined the Cook ART portfolio with Hamilton Thorne, a leading provider of precision instruments, consumables, software, and services to the ART research and the cell biology space.
“Part of achieving our business strategy has included reviewing our product portfolio and identifying what product lines are the best fit for Cook moving forward and which might have more opportunity to grow or thrive elsewhere,” said DJ Sirota, senior vice president of Cook Medical’s MedSurg division.
“This announcement is the conclusion of a series of planned Cook divestures across multiple product lines and specialties to align with Cook’s more focused vision for the future of the company,” said Pete Yonkman, president of Cook Medical and Cook Group.
2. Innovating our training with new product simulators
 If you’ve been to VIVA or VEITH conferences recently, you might have noticed extra attention at the Cook booths. It’s because we brought our new product simulators!
If you’ve been to VIVA or VEITH conferences recently, you might have noticed extra attention at the Cook booths. It’s because we brought our new product simulators!
Product simulators are an important element of Cook’s comprehensive medical education program. These simulators allow residents, fellows and physicians of all experience levels to get a feel—literally—for how our products work. In addition to providing important practice for treating serious disease states, simulators are good for honing technique and getting used to how products work so physicians are prepared to treat patients to the best of their ability.
In addition to the PAD simulators we’ve had for several years, we’ve recently made new product simulators for the Zenith Fenestrated AAA Endovascular Graft and for embolization coils. You can see them at large industry conferences, as well as certain Cook Vista medical education courses. You can sign up to reserve them for a training session.
3. Building World-First Device Testing Machines
Cook is testing the limits of what’s possible with our devices. Currently, there is no commercially available equipment that can test the longevity of certain materials (like superelastic nitinol, often used in vascular medical devices) in certain loading conditions (like heart beats). To create products that are stronger and last longer, we decided to create the world’s first machine to test these materials that experience hundreds of millions of heart-beat loads.

We partnered with Dynatek Labs, a world leader in medical device testing, equipment,services and consulting. Together we created the first fatigue-to-fracture equipment that tests superelastic alloys in cardiac pulsatile loading. This machine simulates the wear and tear a typical nitinol peripheral stent experiences over time, including electrical pulses and physical movement. The machine simulates the conditions until the device starts to fracture. With this data, we can accurately predict how long materials will last and make safer and more durable devices.
The research on the machine was published in ASTM International and you can read the details here.
4. Introducing Cook Leadership
Get to know the leaders at Cook Medical! Our new Meet Our Leaders series introduces key executives at Cook. You’ll get to hear about their background, what they love about Cook and why they’re passionate about what they do. Here are some of the Cook leaders you can get to know:
Be sure to follow Cook Medical on social media and frequently check our newsroom to learn more about Cook’s leaders and how their expertise is shaping the future of medical devices.

We recently welcomed some of our friends from NOVAS to Cook Medical Limerick, where they presented members of our philanthropy team, Cairde Cook, with a mosaic design of a tree, created by clients of the NOVAS service.
This mosaic is not just a beautiful piece of art, but a representation of the collective journey of NOVAS clients and the positive changes they have experienced through their engagement with the NOVAS art therapy programme. It is also a reminder of our commitment to giving back and fostering deep connections in our communities to make a real difference.
Through our recent charity partnership, NOVAS was able to establish an art cabin at Brother Russell House in Limerick, which has become an invaluable space for their clients, offering a range of creative opportunities including painting, drawing, woodwork, and clay, as part of their Trauma Informed Practice (TIP) approach to therapy and rehabilitation.
The art cabin plays a significant role in this approach, offering clients a safe and empowering environment where they can express themselves creatively.
In November 2023, to mark National TIP Day, NOVAS clients and their teachers came together to create a collaborative piece to symbolise Cook Medical’s partnership with NOVAS. The chosen piece was the tree mosaic, representative of the strength, hope and resilience fostered through our collaborative efforts.
“He who plants a tree, plants a hope”, Lucy Larcom, Poet

We are proud to see the impact of our support for the vital work of NOVAS, and to have played a role in creating a space that brings healing, connection and hope to those who need it most.
Cook Medical has announced a new distribution partnership with Movianto UK, a leading European supply chain solutions partner, in the United Kingdom. Starting October 2024, orders from customers in the UK will be shipped from Movianto UK, enhancing Cook Medical’s commitment to better serving customers and delivering life-saving products more efficiently.

One of the key benefits of this partnership is the quicker delivery of Cook products to customers in the UK. Products will now be shipped from within the country, creating a more streamlined and smooth process while reducing the risk of delays.
Additionally, by shipping products by road rather than air, Cook Medical aims to reduce the carbon footprint associated with transportation, contributing to a more sustainable future.
Partnerships like this are just one of the many ways Cook is changing to better serve its customers and patients. As Cook continues to change in line with its vision, an integral part of the plan is fostering partnerships to enhance product offerings.
Speaking about the new partnership, Eamonn Barry, director of Customer Support and Distribution, EMEA at Cook Medical, said, “We are thrilled to collaborate with Movianto UK to expedite the delivery of our products to customers and, ultimately, patients across the UK. Our experience working with Movianto UK has been hugely positive so far. Their values and ethos closely align with those of Cook Medical. We look forward to continuing this successful partnership.”
David Evans, managing director of Movianto UK, added, “Thanks to our specialisation in logistics for the pharmaceutical and healthcare industry, we were able to quickly implement Cook Medical’s wishes. Now, we are delighted that our warehousing and dispatch service provides the basis for next-day delivery to Cook customers nationwide. This is faster and more environmentally friendly than the previous distribution model.”
3,500 people participated in the Cook Medical Mini Marathon at the University of Limerick on Sunday, 6 October.
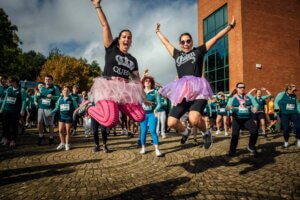
The event welcomed participants from all over the country to run, jog and walk the 5 km and 10 km courses, with many raising money for personally chosen charities. This year’s donations are expected to reach over €50,000.
Commenting on the event, Carla DiBenedetto, HR director and race ambassador at Cook Medical, said, “We are delighted to see so many people taking part and enjoying the Cook Medical Mini Marathon. This event is so special to us, as it not only brings people together for fun and fitness but also raises essential funds for numerous charities. Our commitment to building strong connections within our community is at the core of who we are, and we are truly honoured to be part of this inspiring event.”
Also speaking about the event, Race Director John Cleary said, “This year’s Mini Marathon has been a huge success, bringing together participants of all ages and abilities, which truly embodies the spirit of the race. I would like to thank everyone who took part and all those who supported this event.”

This year marks Cook Medical’s ninth consecutive year sponsoring the Mini Marathon, which has been extended through 2027. The Mini Marathon is just one of the ways Cook connects with its local community in Limerick to contribute to its health and well-being. At many of its sites around the world, Cook is committed to making a positive impact where it operates in its unique Cook way.
Limerick, Ireland — Cook Medical created a custom-made device that was used in the world’s first Frozen Elephant Trunk (FET) aortic procedure that used a company–manufactured fenestrated graft. This groundbreaking procedure demonstrates Cook’s continued commitment to underserved patient populations. As Cook continues to change, we will keep innovating products as well as surgical techniques to better treat patients.

Dr. François Dagenais, a cardiothoracic surgeon based in Quebec City, Canada, who performed the innovative procedure using a Cook custom-made aortic device
Earlier this year, Dr. François Dagenais, a cardiothoracic surgeon based in Quebec City, Canada, approached Cook Medical. One of his patients had severe complex aortic arch disease. He explained that he needed to perform a Frozen Elephant Trunk (FET) procedure on the patient, but he wondered if it could be done with some innovative and different techniques. He asked if Cook could extend to the descending thoracic aorta and include a unique fenestration for the left subclavian artery (LSA).
An FET procedure is a hybrid technique that combines surgically replacing the aortic arch while simultaneously stenting the descending thoracic aorta. Patients requiring FET procedures often have complex aortic arch disease. One of the significant benefits of using an FET procedure is that it enables the physician to complete the aortic repair in a single operation instead of multiple operations.
Although FET procedures have been performed before, one had had never been performed with a custom-made device that had a fenestration. Adding a fenestration in the aortic graft removed the need for surgical intervention for the LSA vessel, allowing for a smooth endovascular LSA stenting and a streamlined, more minimally invasive procedure.
“We performed the world’s first procedure using a fenestrated Frozen Elephant Trunk device. This unique case was designed through Cook Medical’s custom-made device program. During FET procedures, the LSA can be challenging to access surgically. Using an endovascular technique to revascularize the LSA through a pre-designed fenestration within the FET graft facilitates the procedure, decreases operative time and minimizes the complication rate of the FET procedure. The development of this unique FET device is an example of collaboration between clinicians and the expertise of Cook Medical in fenestrated graft technology. Many thanks to Cook Medical for your support; one more step forward in our journey of innovation for the best interest of patient care,” said Dr. Dagenais.
“As Cook Medical continue innovating in the aortic space, our custom-made devices are an area in which we are prioritizing resources” said Johnny LeBlanc, director of Cook’s aortic product management team. “We are committed to collaborating with physicians to address the unmet needs of their patients.”
This case represented a collaborative and innovative partnership between Dr. Dagenais and Cook’s product and engineering teams, which showcased their skills and expertise in custom-made devices. While working with Dr. Dagenais on the custom-made device for the FET procedure, the product and engineering teams used prototypes and 3D anatomical models to evaluate how the device would deploy. This provided an opportunity for fast device improvements which led to the final device specifications.
For decades, Cook Medical have been a leader in custom-made aortic grafts. Clinical specialists, product managers, engineers, and the planning team collaborate directly with physicians to tailor each device to the needs of the patient. For many of the patients who receive custom-made devices, traditional surgical methods are not an option due to the diseased anatomy. Innovative solutions for complex anatomies offer hope for patients who otherwise would have no other treatment options available.
To stay updated on Cook Medical’s latest aortic news, visit https://www.cookmedical.eu/aortic-intervention/.
Dr. Dagenais is a paid consultant of Cook Medical.
About Cook Medical
Since 1963, Cook Medical has worked closely with physicians to develop technologies that eliminate the need for open surgery. Today we invent, manufacture, and deliver a unique portfolio of medical devices to the healthcare systems of the world. Serving patients is a privilege and we demand the highest standards of quality ethics, and service. We have remained family owned so that we have the freedom to focus on what we care about: our patients, our employees, and our communities.
Find out more at CookMedical.eu, and for the latest news, follow us on Twitter, Facebook and LinkedIn.