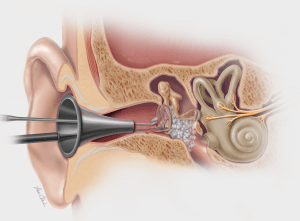
Bloomington, Ind. — Today, Cook Medical announced a new post-market study for the Biodesign® Otologic Repair Graft. Otologic repair on perforated eardrums usually requires physicians to harvest patient tissue from elsewhere in the body to repair the membrane. This Cook product does not require tissue harvesting and instead uses a biomaterial to aid in the natural healing process of the patient’s tissue. The study focuses on closure rates in patients who are treated with the biomaterial.
This is a global study evaluating clinical performance of the Biodesign Otologic Repair Graft. It builds on the several peer-reviewed publications that were single-site studies. By sponsoring this study, Cook continues its tradition of building clinical knowledge to support surgeons’ use of the product. The study will involve 151 patients at six centres in four different countries. Study enrolment is expected to be finished by February 2022.
‘When we started planning for this study, we knew this was a chance to expand data around innovative biologic products and procedures. This study is evidence of Cook’s commitment to minimally invasive procedures. When more data is available around a product or a procedure, Cook can better serve patients’, said Thomas Cherry, director of Cook’s Otolaryngology—Head & Neck Surgery (OHNS) specialty.
The Biodesign Otologic Repair Graft is an extracellular matrix (ECM) graft material for tympanic membrane perforation closure (surgeries to repair the eardrum). Normally, when a patient has a perforated eardrum, traditional surgeries harvest similar tissue from elsewhere in the body and then implant it into the ear. However, this Biodesign Graft can simply be placed in the ear. This can lead to faster procedures for the patient.1 Biodesign Grafts may also mean less scarring for the patient, since there is no need to harvest tissue from elsewhere.2
When implanted, the Biodesign Graft material completely remodels into natural tissue, resulting in closure rates of the eardrum membrane ranging from 86–100% across published literature.1-3 The graft is available in multiple sizes, which can be further cut and adjusted to fit a patient’s unique anatomy.
To learn more about the Biologic Graft or to speak to a representative, visit CookMedical.eu/otolaryngology.
About Cook Medical
Since 1963, Cook Medical has worked closely with physicians to develop technologies that eliminate the need for open surgery. Today we invent, manufacture and deliver a unique portfolio of medical devices to the healthcare systems of the world. Serving patients is a privilege, and we demand the highest standards of quality, ethics and service. We have remained family owned so that we have the freedom to focus on what we care about: patients, our employees and our communities.
Find out more at CookMedical.eu and for the latest news, follow us on Twitter, Facebook and LinkedIn.
1. D’Eredità R. Porcine small intestinal submucosa (SIS) myringoplasty in children: a randomized controlled study. Int J Pediatr Otorhinolaryngol. 2015;79(7):1085–1089.
2. Yawn RJ, Dedmon MM, O’Connell BP, et al. Tympanic membrane perforation repair using porcine small intestinal submucosal grafting. Otol Neurotol. 2018;39(5):e332–e335.
3 De Zinis LO, Berlucchi M, Nassif N. Double-handed endoscopic myringoplasty with a holding system in children: preliminary observations. Int J Pediatr Otorhinolaryngol. 2017;96:127-130.