Bloomington, Ind. — Medical’s Zilver PTX drug-eluting stent (DEShas lower rates of in-stent occlusions among patients with restenosis at three years than Boston Scientific’s Eluvia DES, according to real-world data from the REALDES study. The data, published by Tsuyoshi Shibata, M.D., Ph.D., et al., in the European Journal of Vascular & Endovascular Surgery (EJVES) are the first to compare Zilver PTX and Eluvia in real-world practice at three years.1 These data stand as another proof point of Cook’s relentlessly innovative products.
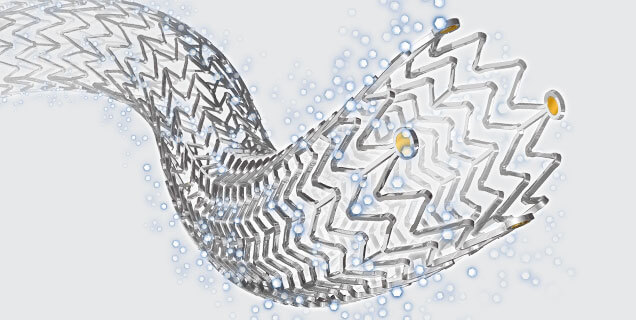
Zilver® PTX®, Cook Medical’s paclitaxel-coated stent
“There have been many unanswered questions on the reliability of the current data comparing the different drug-eluting stents on the market. REALDES helps paint a more complete picture on DES differences, evaluated by independent investigators in a real-world setting,” said Alec Cerchiari, director of product management for Cook’s PAD & Venous specialty. “We are grateful to the physicians and hospitals that participated in this study.”
REALDES is an investigator-initiated, multi-centre, prospective, observational study designed to compare Cook’s Zilver PTX DES and Boston Scientific’s Eluvia DES in a real-world setting for treating symptomatic femoropopliteal lesions. Overall, 200 limbs with native femoropopliteal artery disease were treated with Zilver PTX (96 limbs) or Eluvia (104 limbs) at eight Japanese hospitals between February 2019 and September 2020. The primary outcome measure of this study was primary patency at three years, defined as freedom from restenosis* or occlusion without reintervention.
Key three-year study outcomes include:
- There was no significant difference in primary patency (Zilver PTX, 70.0% vs. Eluvia, 65.2%; P = 0.74) or freedom from CD-TLR (79.4% vs. 76.3%; P = 0.27), despite the Zilver PTX arm having longer lesions (185.7 ± 92 mm vs. 160.0 ± 99 mm; P < .005).
- In patients with restenosis at three years, there was a significantly higher rate of in-stent occlusions (Tosaka class III2) for those treated with Eluvia (57.7%) compared to Zilver PTX (29.2%; P = 0.041).
“These findings contribute valuable insights into optimising treatment outcomes and guiding future stent selection,” said lead author Dr. Shibata of Sapporo Medical University.
To learn more about Zilver PTX, visit Zilver PTX | Cook Medical.
About Cook Medical
At Cook Medical, we are passionate about making unique, quality medical devices and connecting with people to improve lives. Founded on inventing, manufacturing, and delivering medical devices, we provide healthcare professionals with the tools they need to help their patients return to living.
Our commitment to innovation involves bringing new products to market and keeping existing products relevant to a changing healthcare landscape. We believe in using our business to help people and communities thrive by creating inclusive, supportive, and healthy environments.
We are proud of our history of innovative firsts and the impact we have on patients and communities. With headquarters in Bloomington, Indiana, and manufacturing facilities and offices in various global locations, we challenge ourselves to maintain a global perspective while focusing on local impact.
Follow Cook Medical at CookMedical.eu and on LinkedIn.
*Restenosis was characterised as peak systolic velocity ratio > 2.4 based on the duplex ultrasonography findings or the recurrence of ≥50% diameter stenosis as determined by angiographic findings.
1 Shibata T, Iba Y, Shingaki M, et al. Comparative analysis of three-year results of two paclitaxel related stents for the management of femoropopliteal disease in a real-world setting. Eur J Vasc Endovasc Surg.2025; doi: 10.1016/j.ejvs.2025.03.010
2 Tosaka A, Soga Y, Iida O, et al. Classification and clinical impact of restenosis after femoropopliteal stenting. J Am Coll Cardiol. 2012;59:16-23.
Our mission stays the same, but at Cook, we’re relentlessly inventive and proactively adapting to the needs of physicians and patients.
We previously shared three ways Cook is changing. Since then, we’ve made other significant advancements. Here are four more ways Cook is changing to better serve our customers.
1. Growing our product portfolio to fit our future
We’ve recently adjusted our product portfolio to better offer products that align with our vision and serve unmet customer needs with a unique portfolio of products. These portfolio changes include:
Investing in Zenflow
Cook Medical announced a strategic investment in the urology space. Zenflow is a medical device company developing a minimally invasive treatment for urinary obstruction caused by enlarged prostate, or benign prostatic hyperplasia (BPH).“This investment reflects our confidence in the future of Zenflow and the Spring® System technology. Zenflow is aligned with our core focus of developing minimally invasive technologies that restore flow,” said DJ Sirota, senior vice president of Cook Medical’s MedSurg division. “This type of agreement is yet another proof-point to how Cook is changing to focus on our future as product innovators.”
Investing in PillSense
Cook Medical signed an agreement to distribute EnteraSense’s novel blood-sensing capsule, PillSense. We also invested in EnteraSense’s Series B round of funding to support the production and distribution of PillSense capsules. PillSense is a strategic addition to Cook’s portfolio of endoscopic bleed management products, such as Hemospray and the Instinct Plus™ Endoscopic Clipping Device.
Divesting Lead Management products
Cook Medical sold our portfolio of lead management products to Merit Medical. Merit has an existing footprint in the electrophysiology (EP) space and plans to expand their presence in Lead Management. Merit is making significant investment in EP and shows they are committed to growing the business in ways that Cook has not been able to in recent years. This acquisition represents a tremendous opportunity for employees, products and patients while aligning with Cook’s long-term goals.
Divesting the Reproductive Health portfolio
Cook Medical sold our portfolio of Reproductive Health and Assisted Reproductive Technology (ART) products to Astorg. Astorg, an investment firm with deep experience in MedTech across both manufacturing and product businesses with a focus on growth and innovation, acquired and simultaneously combined the Cook ART portfolio with Hamilton Thorne, a leading provider of precision instruments, consumables, software, and services to the ART research and the cell biology space.
“Part of achieving our business strategy has included reviewing our product portfolio and identifying what product lines are the best fit for Cook moving forward and which might have more opportunity to grow or thrive elsewhere,” said DJ Sirota, senior vice president of Cook Medical’s MedSurg division.
“This announcement is the conclusion of a series of planned Cook divestures across multiple product lines and specialties to align with Cook’s more focused vision for the future of the company,” said Pete Yonkman, president of Cook Medical and Cook Group.
2. Innovating our training with new product simulators
 If you’ve been to VIVA or VEITH conferences recently, you might have noticed extra attention at the Cook booths. It’s because we brought our new product simulators!
If you’ve been to VIVA or VEITH conferences recently, you might have noticed extra attention at the Cook booths. It’s because we brought our new product simulators!
Product simulators are an important element of Cook’s comprehensive medical education program. These simulators allow residents, fellows and physicians of all experience levels to get a feel—literally—for how our products work. In addition to providing important practice for treating serious disease states, simulators are good for honing technique and getting used to how products work so physicians are prepared to treat patients to the best of their ability.
In addition to the PAD simulators we’ve had for several years, we’ve recently made new product simulators for the Zenith Fenestrated AAA Endovascular Graft and for embolization coils. You can see them at large industry conferences, as well as certain Cook Vista medical education courses. You can sign up to reserve them for a training session.
3. Building World-First Device Testing Machines
Cook is testing the limits of what’s possible with our devices. Currently, there is no commercially available equipment that can test the longevity of certain materials (like superelastic nitinol, often used in vascular medical devices) in certain loading conditions (like heart beats). To create products that are stronger and last longer, we decided to create the world’s first machine to test these materials that experience hundreds of millions of heart-beat loads.

We partnered with Dynatek Labs, a world leader in medical device testing, equipment,services and consulting. Together we created the first fatigue-to-fracture equipment that tests superelastic alloys in cardiac pulsatile loading. This machine simulates the wear and tear a typical nitinol peripheral stent experiences over time, including electrical pulses and physical movement. The machine simulates the conditions until the device starts to fracture. With this data, we can accurately predict how long materials will last and make safer and more durable devices.
The research on the machine was published in ASTM International and you can read the details here.
4. Introducing Cook Leadership
Get to know the leaders at Cook Medical! Our new Meet Our Leaders series introduces key executives at Cook. You’ll get to hear about their background, what they love about Cook and why they’re passionate about what they do. Here are some of the Cook leaders you can get to know:
Be sure to follow Cook Medical on social media and frequently check our newsroom to learn more about Cook’s leaders and how their expertise is shaping the future of medical devices.
Limerick, Ireland — Cook Medical created a custom-made device that was used in the world’s first Frozen Elephant Trunk (FET) aortic procedure that used a company–manufactured fenestrated graft. This groundbreaking procedure demonstrates Cook’s continued commitment to underserved patient populations. As Cook continues to change, we will keep innovating products as well as surgical techniques to better treat patients.

Dr. François Dagenais, a cardiothoracic surgeon based in Quebec City, Canada, who performed the innovative procedure using a Cook custom-made aortic device
Earlier this year, Dr. François Dagenais, a cardiothoracic surgeon based in Quebec City, Canada, approached Cook Medical. One of his patients had severe complex aortic arch disease. He explained that he needed to perform a Frozen Elephant Trunk (FET) procedure on the patient, but he wondered if it could be done with some innovative and different techniques. He asked if Cook could extend to the descending thoracic aorta and include a unique fenestration for the left subclavian artery (LSA).
An FET procedure is a hybrid technique that combines surgically replacing the aortic arch while simultaneously stenting the descending thoracic aorta. Patients requiring FET procedures often have complex aortic arch disease. One of the significant benefits of using an FET procedure is that it enables the physician to complete the aortic repair in a single operation instead of multiple operations.
Although FET procedures have been performed before, one had had never been performed with a custom-made device that had a fenestration. Adding a fenestration in the aortic graft removed the need for surgical intervention for the LSA vessel, allowing for a smooth endovascular LSA stenting and a streamlined, more minimally invasive procedure.
“We performed the world’s first procedure using a fenestrated Frozen Elephant Trunk device. This unique case was designed through Cook Medical’s custom-made device program. During FET procedures, the LSA can be challenging to access surgically. Using an endovascular technique to revascularize the LSA through a pre-designed fenestration within the FET graft facilitates the procedure, decreases operative time and minimizes the complication rate of the FET procedure. The development of this unique FET device is an example of collaboration between clinicians and the expertise of Cook Medical in fenestrated graft technology. Many thanks to Cook Medical for your support; one more step forward in our journey of innovation for the best interest of patient care,” said Dr. Dagenais.
“As Cook Medical continue innovating in the aortic space, our custom-made devices are an area in which we are prioritizing resources” said Johnny LeBlanc, director of Cook’s aortic product management team. “We are committed to collaborating with physicians to address the unmet needs of their patients.”
This case represented a collaborative and innovative partnership between Dr. Dagenais and Cook’s product and engineering teams, which showcased their skills and expertise in custom-made devices. While working with Dr. Dagenais on the custom-made device for the FET procedure, the product and engineering teams used prototypes and 3D anatomical models to evaluate how the device would deploy. This provided an opportunity for fast device improvements which led to the final device specifications.
For decades, Cook Medical have been a leader in custom-made aortic grafts. Clinical specialists, product managers, engineers, and the planning team collaborate directly with physicians to tailor each device to the needs of the patient. For many of the patients who receive custom-made devices, traditional surgical methods are not an option due to the diseased anatomy. Innovative solutions for complex anatomies offer hope for patients who otherwise would have no other treatment options available.
To stay updated on Cook Medical’s latest aortic news, visit https://www.cookmedical.eu/aortic-intervention/.
Dr. Dagenais is a paid consultant of Cook Medical.
About Cook Medical
Since 1963, Cook Medical has worked closely with physicians to develop technologies that eliminate the need for open surgery. Today we invent, manufacture, and deliver a unique portfolio of medical devices to the healthcare systems of the world. Serving patients is a privilege and we demand the highest standards of quality ethics, and service. We have remained family owned so that we have the freedom to focus on what we care about: our patients, our employees, and our communities.
Find out more at CookMedical.eu, and for the latest news, follow us on Twitter, Facebook and LinkedIn.

Cook Medical’s Resonance® Metallic Ureteral Stent
Bloomington, Ind. — The British Journal of Urology International Compass published a health economics model showing the benefits of Cook Medical’s Resonance® Metallic Ureteral Stent when used as a first-line treatment option for extrinsic malignant ureteric obstruction (MUO). The research demonstrates that the stent is a win for both health systems and patients: the stent is more cost-effective than standard polyurethane ureteral stents, as well as offering several health benefits to patients.
This newly published health economic analysis sought to assess whether the use of polyurethane double-J (JJ) stents or the Resonance metallic stent is more cost-effective for managing extrinsic MUO in the UK healthcare setting. Malignant ureteral obstruction is a frequent challenge for urologists. Patients with MUO have poor prognoses, and treatment usually aims to improve quality of life while optimising renal function.
Currently, the standard practice is to treat MUO using polyurethane stents, which require frequent surgical replacements due to blockages and encrustation. Metallic stents are more durable, but they have a higher initial purchase price, which makes healthcare systems wary of them. This ecomomic report shows that even though metallic stents have a higher up-front cost, they tend to be more cost-effective over time. Here are some of the cost benefits that the report showed:
- Use of the Resonance stent is likely to result in between a £726- £1075 saving per patient within year 1.1
- Treatment of MUO with Resonance incurs 23.4% less overall treatment costs than polyurethane stents over 5 years.1
- By removing unnecessary, unplanned surgical re-intervention, the use of Resonance may free up surgical capacity for the elective provision and reduce the burden on the emergency readmission due to stent failure.1
In addition to demonstrating that the Resonance stent is cost-effective, the report also showed several health benefits to patients. While it is important to consider affordability and healthcare economics, the main goal of treatment is improving the quality of life for the patient. The report showed the following patient benefits:
- Resonance use in MUO predicts 2 fewer surgical interventions within 1 year per patient.1
- Patients experienced increased quality of life due to reduced stent failure and the associated pain and impact of further surgical intervention.1
- Resonance stents increase the functional duration by 4 months2 and have a lower risk of stent failure.1
- Physicians and patients who used the Resonance stent avoided stent exchanges needed due to failure or encrustation. Stent exchange can be technically difficult, may fail, can cause complications with added morbidity, and may itself further compromise the patient’s quality of life.3 4
“We want to show physicians that investing in higher-quality medical devices is worth it,” said Johan Lowinger, director of product management for Cook’s Urology specialty. “Research like this is about more than just data; it’s about the rethinking the implications of treatment plans, physician practices and patient outcomes. When we prioritize patient health and put people first, it’s a win-win situation for everyone involved: patients, physicians and health systems alike.”
To learn more about the Resonance Metallic Ureteral Stent, you can view product details here.
About Cook Medical
Since 1963, Cook Medical has worked closely with physicians to develop technologies that eliminate the need for open surgery. Today we invent, manufacture, and deliver a unique portfolio of medical devices to the healthcare systems of the world. Serving patients is a privilege and we demand the highest standards of quality ethics, and service. We have remained family owned so that we have the freedom to focus on what we care about: our patients, our employees, and our communities.
Find out more at CookMedical.eu, and for the latest news, follow us on Twitter, Facebook and LinkedIn.
1 Cooper DM, Lines R and Shergill I. Cost-Effectiveness of Resonance® metallic ureteral stent compared with standard polyurethane ureteral stents in malignant ureteric obstruction: a cost-utility analysis. BJUICompas 2024; Volume issues page number and doi TBC.
2 Chow PM, Chiang IN, Chen CY, Huang KH, Hsu JS, Wang SM, et al. Malignant Ureteral Obstruction: Functional Duration of Metallic versus Polymeric Ureteral Stents. PLoS One. 2015/08/13. 2015;10(8):e0135566.
3 Khoo CC, Abboudi H, Cartwright R, El-Husseiny T, Dasgupta R. Metallic Ureteric Stents in Malignant Ureteric Obstruction: A Systematic Review. Urology. 2018 Aug;118:12–20.
4 Sountoulides P, Kaplan A, Kaufmann OG, Sofikitis N. Current status of metal stents for managing malignant ureteric obstruction. BJU Int. 2010 Apr;105(8):1066–72.
On 5 February, the Medicines and Healthcare products Regulatory Agency (MHRA) made a critical announcement: the agency updated their guidance on the use of paclitaxel-coated devices (PCDs).
After carefully reviewing available evidence and receiving input from an independent expert working group, the MHRA updated their previous advice on PCDs to remove restrictions on indication, dose, and repeated exposure. This meant withdrawing its previous advice to use the lowest dose PCDs available and to avoid/reduce repeated exposure of paclitaxel coated devices is withdrawn.
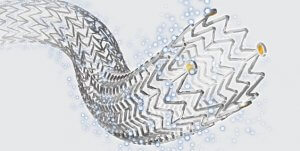
Cook Medical’s Zilver PTX product
Additionally, they affirmed that “where indicated, PCD can be considered a treatment option in patients with critical limb ischaemia (CLI) or intermittent claudication (IC).”
In July 2023, the U.S. Food and Drug Administration (FDA) concluded there is no increased mortality risk for paclitaxel devices. Additionally, Health Canada and the Therapeutic Goods Association (TGA) of Australia have concluded there is no increased risk of mortality associated with the use of paclitaxel devices. Each of these organizations made their decision following review of available evidence, including an updated meta-analysis of the randomized studies, which Cook supported. Cook Medical applauds the updates from all of these regulatory agencies. We believe these updates are in the best interest of both physicians and patients.
“We are grateful for the MHRA’s latest update on paclitaxel. They carefully reviewed the data, and they did so with patients’ best interests in mind,” said Mark Breedlove, senior vice president of Cook Medical’s Vascular division. “Paclitaxel-coated devices, such as Cook Medical’s Zilver® PTX® Drug-Eluting Peripheral Stent, benefit millions of patients worldwide who are suffering from peripheral artery disease (PAD). We make data-based decisions about our procedural solutions, and we believe the MHRA has made the correct decision in lifting the restrictions.”
Concerns around paclitaxel first started circulating in 2018 from a meta-analysis conducted by Dr. Konstantinos Katsanos, et al. The analysis suggested there was an increased mortality risk associated with paclitaxel-coated devices.
Understandably, the entire medical device industry became invested. A few months later, the United States Food and Drug Administration (FDA) released its initial warning. Although the products from all medical device manufacturers would remain on the market, the letter said that physicians should be aware that paclitaxel could be potentially associated with increased mortality. The letter noted that Katsanos, et. al’s research should be “interpreted with caution because of multiple limitations,” but the FDA needed to do its due diligence and investigate further.
In the spirit of transparency, Cook released five-year patient-level data from the Zilver PTX randomized control trial—and we were the first and only company to be completely transparent with our data. Zilver PTX is a drug-eluting stent indicated to treat vascular disease of the above-the-knee femoropopliteal arteries. On Cook’s website, physicians and organizations could request the de-identified patient information at no charge. We also worked tirelessly with physicians from multiple regulatory organizations to understand the truth.
Cook will continue providing Zilver PTX. We will continue to make decisions based on evidence as the most reliable way to treat patients. We also reaffirm our commitment to transparency around data.
To see more data on paclitaxel, Zilver PTX presentations at conferences and other related information, you can visit our paclitaxel page.
Limerick, Ireland — Cook Medical’s Advance Serenity® Hydrophilic PTA Balloon Dilation Catheter is now available with even more size options in Europe. In June, interventionalists who perform peripheral intervention procedures in the US and Canada got access to more sizes of the Advance Serenity product to treat patients with peripheral artery disease (PAD), and Cook is excited to expand those options to the EU as well.
Cook Medical’s Advance Serenity 18 product
Now, Advance Serenity is available to help physicians treat complex lesions with more balloon sizes. Advance Serenity is still made with its laser-formed, low-profile entry tip. The next-generation hydrophilic coating provides lubricity to navigate through diseased vessels with reduced friction. The Advance Serenity 18 range now includes balloon diameters from 5mm to 10mm and is compatible with 4 and 5 Fr sheaths to help physicians treat complex lesions with options to include larger-vessel PAD. The Advance Serenity 14 and Serenity 18 balloons are still available in 2mm–4mm balloon diameters with 4 Fr sheath compatibility.
“PAD affects around 113 million people.1 If left untreated, PAD can become deadly,” said Alec Cerchiari, director of product management for Cook’s PAD and Venous Specialty. “After looking at the needs of patients with this condition, we built on our previous innovation and are excited to expand the sizes, uses and availability of this product. The Serenity balloon complements our balloon portfolio for interventionalists who are treating patients with complex lesions.”
The catheter is manufactured by Surmodics and distributed by Cook Medical. You can learn more about the Serenity product here.
About Cook Medical
Since 1963, Cook Medical has worked closely with physicians to develop technologies that eliminate the need for open surgery. Today we invent, manufacture and deliver a unique portfolio of medical devices to the healthcare systems of the world. Serving patients is a privilege, and we demand the highest standards of quality, ethics and service. We have remained family-owned so that we have the freedom to focus on what we care about: patients, our employees and our communities.
Find out more at CookMedical.eu and for the latest news, follow us on Twitter, Facebook and LinkedIn.
1. Lin, Jinfeng, Yangbo Chen, Nan Jiang, Zuoshi Li, and Shangbo Xu. “Burden of peripheral artery disease and its attributable risk factors in 204 countries and territories from 1990 to 2019.” Frontiers in Cardiovascular Medicine 9 (2022).
Cook applauds the U.S. Food and Drug Administration’s recent update on paclitaxel-coated devices. We believe this is the best decision for physicians and patients.
“We are grateful for the FDA’s latest update on paclitaxel. We applaud decisions that are based on long-term, patient-level data,” said Mark Breedlove, senior vice president of Cook Medical’s Vascular division. “Paclitaxel-coated devices, such as Cook Medical’s Zilver® PTX® Drug-Eluting Peripheral Stent, benefit millions of patients suffering from peripheral artery disease (PAD). We make data-based decisions about our procedural solutions, and we believe the FDA made the right call for patients.”

Zilver® PTX®, Cook Medical’s paclitaxel-coated stent
On July 11, 2023, the FDA released a long-awaited letter to healthcare providers announcing “the data does not support an excess mortality risk for paclitaxel-coated devices.”
This announcement nullifies the FDA’s earlier recommendation that physicians use caution with paclitaxel-coated devices because of a possible unnecessary mortality risk. With this new statement the FDA agrees that paclitaxel and paclitaxel-coated devices are safe and effective for patients suffering from peripheral artery disease (PAD).
Concerns around paclitaxel started circulating in 2018 from meta-analysis conducted by Dr. Konstantinos Katsanos, et al. The analysis suggested there was an increased mortality risk associated with paclitaxel-coated balloons.
Understandably, the entire medical device industry became invested. A few months later, the FDA released its initial warning. Although the products from all medical device manufacturers would remain on the market, the letter said that physicians should be aware that paclitaxel could be potentially associated with increased mortality. The letter noted that Katsanos, et. al’s research should be “interpreted with caution because of multiple limitations,” but the FDA needed to do its due diligence and investigate further.
In the spirit of transparency, Cook released five-year patient-level data from the Zilver PTX randomized control trial—and we were the first and only company to be completely transparent with our data. Zilver PTX is a drug-eluting stent indicated to treat vascular disease of the above-the-knee femoropopliteal arteries. On Cook’s website, physicians and organizations could request the de-identified patient information at no charge. We also worked tirelessly with physicians and with the FDA to understand the truth.
In September 2019, Dr. Michael Dake, et al. published a peer-reviewed article analyzing patient-level data from two large studies with long-term follow-up to determine whether there was an increased mortality risk due to paclitaxel. The results demonstrated no increase in long-term all-cause mortality. Cook has repeatedly defended our technology with data at various conferences and in a study published in the Journal of Vascular and Interventional Radiology.
In July 2023, after a thorough investigation, the FDA released a statement saying, “Based on the FDA’s review of the totality of the available data and analyses, we have determined that the data does not support an excess mortality risk for paclitaxel-coated devices.”
Cook will continue providing Zilver PTX. We will continue to make decisions based on evidence as the most reliable way to treat patients. We also reaffirm our commitment to transparency around data.
To see more data on paclitaxel, Zilver PTX presentations at conferences and other related information, you can visit our paclitaxel page.
About Cook Medical
Since 1963, Cook Medical has worked closely with physicians to develop technologies that eliminate the need for open surgery. Today we invent, manufacture and deliver a unique portfolio of medical devices to the healthcare systems of the world. Serving patients is a privilege, and we demand the highest standards of quality, ethics and service. We have remained family owned so that we have the freedom to focus on what we care about: patients, our employees and our communities.
Find out more at CookMedical.eu and for the latest news, follow us on Twitter, Facebook and LinkedIn.
Bloomington, Ind. — Following the publication of an animal study examining the performance of embolisation coils in arteries1 in the Journal of Vascular and Interventional Radiology (JVIR) in 2019, a second, similar study,2 published in the May 2023 issue of JVIR, examined fibred and non-fibred embolisation coil performance, this time in the venous system.

Cook Medical’s fibred Nester coil, left, is compared to three non-fibred coils
In this newly published venous ovine study, fibred Nester® coils and non-fibred coils were deployed in 24 veins in 6 sheep. The study’s data confirmed that fibred coils demonstrate several advantages over non-fibred coils. These advantages include the following:
- Fibres significantly boosted the immediate occlusive capacity of coils. In the study, fibred coils achieved stasis in 5.3 minutes, while non-fibred coils took 9.0 minutes.2
- A significantly lower average radiation dose was required when fibred coils were used: 25.3 mGy for fibred coils compared with 34.9 mGy for non-fibred coils.2
The venous fibre study also substantiated the findings of the earlier arterial fibre study: The two studies found that fewer fibred coils are needed to achieve acute occlusion compared to bare metal coils.1, 2 Both studies confirmed that significantly less coil length is needed if the coil is fibred.1, 2
- In the venous fibre study, on average 5 fibred coils, or 70 cm of fibred coil length, were needed to achieve occlusion compared with 8.75 non-fibred coils, or 122.5 cm of bare metal coil length.2
- In the arterial fibre study, on average 1.3 fibred coils, or 9.1 cm of coil length, were required to achieve occlusion, compared to 2 non-fibred coils, or 22.4 cm of non-fibred coil length, that was needed to obtain occlusion.1
Should the study results translate to the human experience, patients treated with fibred coils could potentially experience shorter procedure times, require fewer implants and have less radiation exposure to achieve the same outcomes.*
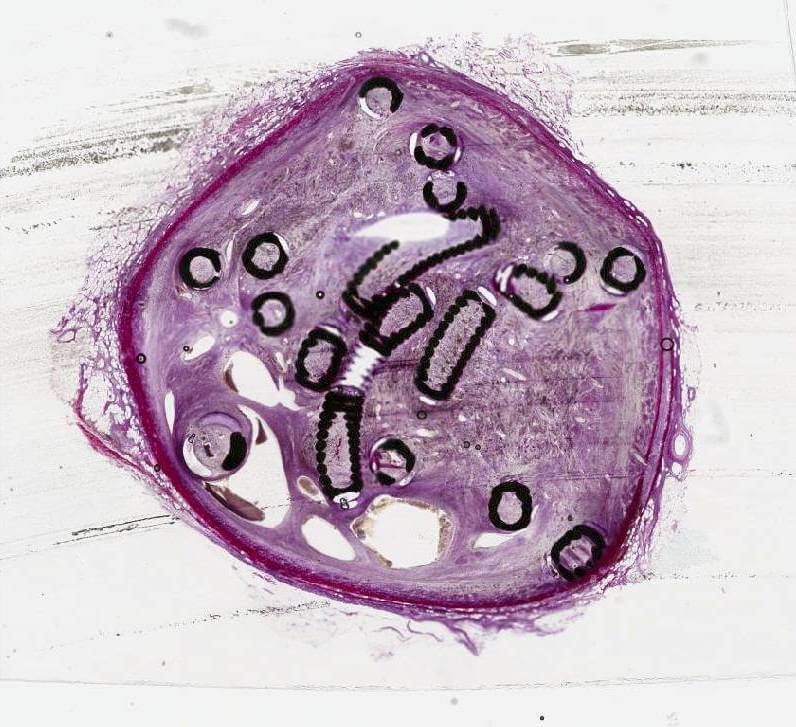
This study used a unique method of generating histologic images of metallic implants by cutting 5µm thin sections of tissue embedded in resin.
In this venous fibre study, a new approach was also applied to generating histologic images. A unique method of cutting 5 µm thin sections of tissue with metallic implants was developed by Cook Research Incorporated (CRI) and Alizée Pathology. Thin sectioning results in clearer and more detailed histologic images, allowing a pathologist to read and interpret tissue changes. An image showing a 5 µm thick microscope slide of Cook embolisation coils in an ovine vein can be seen on the front cover of the May 2023 issue of JVIR and online at www.jvir.org.
“When we see in vivo results like this in studies, we hope the same benefits will be applicable to human patients,” said Remco van der Meel, director of product management for Cook’s Interventional specialty. “We are constantly evaluating our Embolisation portfolio, exploring cutting-edge technology and gathering data to create new technology. Additionally we want to understand how we could best support our customers in achieving the best possible outcomes for patients. This study helps us make data-based decisions on how to best meet the needs for effective embolisation in a cost-efficient manner, while reducing exposure to ionizing radiation for both doctor and patient. These are key elements in today’s healthcare environment.”
To read the full venous animal fibre study, view the article on JVIR’s website. To read the full arterial fibre study article, go here. To learn more about fibred coils, visit our Cook Medical embolisation web page here.
*Definitive conclusions regarding the safety or effectiveness of fibred coils in humans cannot be directly drawn from the results of these animal studies.
About Cook Medical
Since 1963, Cook Medical has worked closely with physicians to develop technologies that eliminate the need for open surgery. Today we invent, manufacture, and deliver a unique portfolio of medical devices to the healthcare systems of the world. Serving patients is a privilege and we demand the highest standards of quality ethics, and service. We have remained family owned so that we have the freedom to focus on what we care about: our patients, our employees, and our communities.
Find out more at CookMedical.eu, and for the latest news, follow us on Twitter, Facebook and LinkedIn.
1 Trerotola SO, Pressler GA, Premanandan C. Nylon fibered versus non-fibered embolization coils: comparison in a swine model. J Vasc Interv Radiol. 2019;30(6):949–955.
2 White SB, Wissing ER, Van Alstine WG, et al. Comparison of fibered versus nonfibered coils for venous embolization in an ovine model. J Vasc Interv Radiol. 2023;34(5):888–895.










