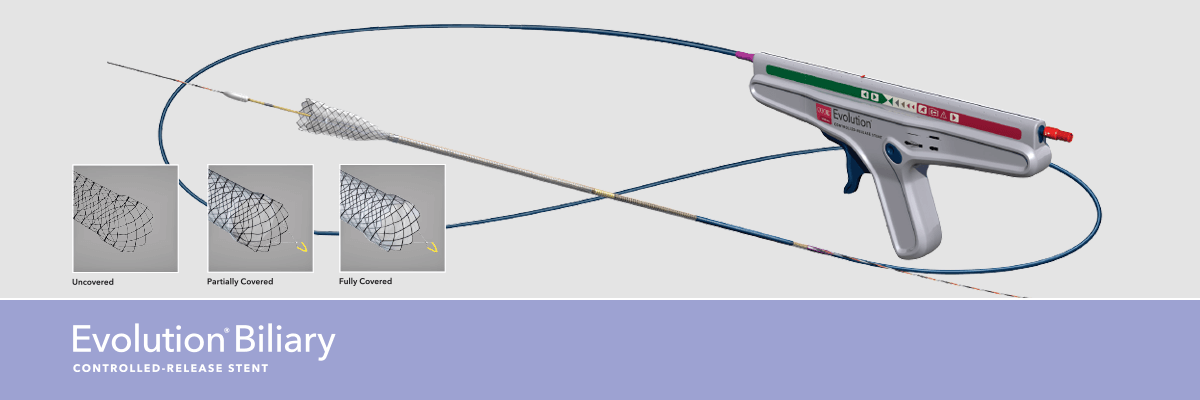

This recently published study evaluates the performance characteristics of the Evolution® Biliary Stent System – Uncovered for the palliation of malignant biliary obstruction. The full clinical article is open access and available online here.
‘The primary study endpoint, clinical success, defined as freedom from symptomatic recurrent biliary obstruction (within the stent) requiring re-intervention, was achieved in 108 of 113 patients (95.6, 95%CI = 90.0–98.6%) at study exit for each patient.’
‘Technical success [defined as ‘successful delivery and placement of a stent at its intended location’] was achieved in 112/113 patients (99.1%, CI 95% = 95.1–100.0%), and in 119/120 stent placements (99.2%).’
The study concludes that the product ‘appears to be effective at relieving biliary obstruction and preventing re-intervention within 6 months of insertion in the overwhelming majority of patients. The device has an excellent safety profile, and possess[es] a high technical success rate during deployment in a variety of types of biliary strictures.’
The product information on this website is intended only for physicians and healthcare professionals licensed in the European Union (except France), the United Kingdom, Switzerland, Norway, Iceland, Turkey, and Liechtenstein. If you are located in another global region, please click on the regional flag at the very top of the webpage and choose your region from the drop-down options.
If your region is not listed, visit our How to Order section for more information.
Information provided on this site is not intended to be professional medical advice. Product Instructions for Use (IFU) should be consulted before use of any product.