Contact A Representative
Since 1963, Cook Medical has been a champion of minimally invasive treatment options for patients. In the case of the centesis and drainage procedural areas, the healthcare industry discussion has largely centred around the efficacy of large-bore versus small-bore catheters.
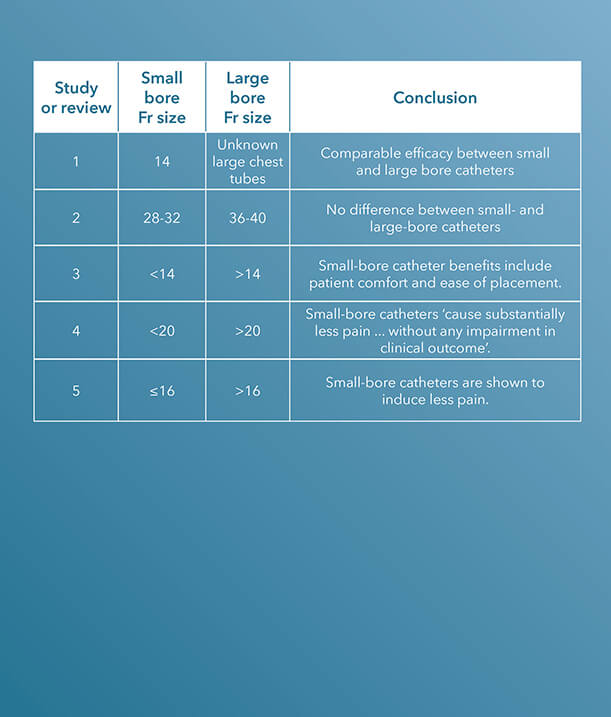
Within the last decade, there have been several clinical studies and review articles published in peer-reviewed journals stating the increased efficacy and improvement of small-bore catheters versus large-bore catheters for pleural and pericardial drainage procedures.
To see Critical Care’s entire product portfolio of small-bore chest tubes for pleural and pericardial drainage, click here.
ARTICLE 1 (STUDY)
‘Two-year experience of using pigtail catheters to treat traumatic pneumothorax: a changing trend’, published in The Journal of Trauma, in 2011, is a study comparing small-bore, 14 Fr pigtail catheters to chest tubes in patients with nontraumatic pneumothoraces.
A chest tube, ‘because of its large caliber and significant trauma during an insertion, can cause pain, prevent full lung expansion, and worsen pulmonary outcome. Pigtail catheters (PC) are smaller and less invasive’, the introduction to the study states.¹
The charts of 9,624 trauma patients over a two-year period at a Level I trauma centre were evaluated. The study concludes that the ‘demographics, tube days, need for mechanical ventilation, and insertion-related complications were similar’, using these characteristics to determine that pigtail catheters have a comparable efficacy to chest tubes.1
ARTICLE 2 (STUDY)
36 to 40 Fr chest tubes were also compared to small-bore pigtail catheters in a similar study also published in The Journal of Trauma and Acute Care Surgery, titled ‘Does size matter? A prospective analysis of 28-32 versus 36-40 French chest tube size in trauma’.
There was no difference in the efficacy of drainage [or] rate of complications.2
In this study, the authors claim that ‘the optimal chest tube size for the drainage of traumatic hemothoraces and pneumothoraces is unknown.’2 This study was designed to compare the efficacy of small-bore versus large-bore drainage catheters in patients with thoracic trauma.
Data were gathered on 293 patients who required open chest tube drainage within 12 hours of admission to a Level I trauma centre.
‘Clinical demographic data and outcomes including efficacy of drainage, complications, retained hemothoraces, residual pneumothoraces, need for additional tube insertion, video-assisted thoracoscopy, and thoracotomy were collected and analyzed by tube size’, the authors indicate.2 Small-bore catheters, ranging from 28 to 32 Fr, were compared with large chest tubes, ranging from 36 to 40 Fr; a total of 353 catheters, 186 small and 167 large, were placed during the study.
The authors determined that ‘the need for tube reinsertion, image-guided drainage, video-assisted thoracoscopy, and thoracotomy’ are the same when comparing small-bore to large-bore catheters.2
‘For injured patients with chest trauma, chest tube size did not impact the clinically relevant outcomes tested. There was no difference in the efficacy of drainage, rate of complications including retained hemothorax, need for additional tube drainage, or invasive procedures’, the study concludes.2
ARTICLE 3 (REVIEW)
In 2013, a paper published in Clinics in Chest Medicine aimed to generalise the use of small-bore catheters for drainage procedures, including in pleural infection.
In ‘Straightening out chest tubes: what size, what type, and when’, the authors state that ‘small-bore tubes (<14 Fr) are effective for most pleural processes. Various types of pneumothorax and malignant and infected complicated pleural effusions have been successfully managed with small-bore chest tubes.’3
Abundant literature supports a paradigm shift towards the more routine use of small-bore chest tubes for managing pleural disease.3
The authors also state that the benefits of using small-bore drainage catheters include patient comfort and ease of catheter placement.3
The study concludes that ‘abundant literature supports a paradigm shift towards the more routine use of small-bore chest tubes for managing pleural disease.’3
ARTICLE 4
A study published in CHEST, titled ‘The relationship between chest tube size and clinical outcome in pleural infection’, aimed to determine the optimal choice of drainage catheter sizes for pleural infection.
This multicentre study enrolled 405 patients and evaluated ‘the combined frequency of death and surgery, and secondary outcomes’, specifically ‘hospital stay, change in chest radiograph, and lung function at 3 months’, in patients who received a variety of sizes of chest tubes. In 128 of the 405 patients, a pain scale was also included.4
‘There was no significant difference in the frequency with which patients either died or required thoracic surgery in patients receiving chest tubes of varying sizes’, the study notes; however, ‘pain scores were substantially higher in patients receiving (mainly blunt dissection inserted) larger tubes’.4
The study concludes that ‘smaller, guide-wire-inserted chest tubes cause substantially less pain than blunt-dissection-inserted larger tubes, without any impairment in clinical outcome in the treatment of pleural infection.’4
ARTICLE 5 (REVIEW)
Although the precise optimal chest drain size remains unknown, a review article titled ‘Optimal chest drain size: the rise of the small-bore pleural catheter’, published in Seminars in Respiratory and Critical Care Medicine, asserts that ‘objective data supporting the use of large-bore [chest] tubes is scarce’.5
The onus now is on those who favor large tubes to produce clinical data to justify the more invasive approach.5
In support of the efficacy of small-bore drains, the article states, ‘Increasing evidence shows that small-bore catheters induce less pain and are of comparable efficacy to large-bore tubes, including in the management of pleural infection, malignant effusion, and pneumothoraces. The onus now is on those who favor large tubes to produce clinical data to justify the more invasive approach.’5
Interested in chatting with a Cook Medical representative?
Please submit the required information to connect with your local Cook representative. This form is intended for US-based physicians only. Please see our Privacy Statement for data protection notices relating to our collection and use of your data.
1. Kulvatunyou N, Vijayasekaran A, Hansen A, et al. Two-year experience of using pigtail catheters to treat traumatic pneumothorax: a changing trend. J Trauma. 2011;71(5):1104-1107.
2. Inaba K, Lustenberger T, Recinos G, et al. Does size matter? A prospective analysis of 28-32 versus 36-40 French chest tube size in trauma. J Trauma Acute Care Surg. 2012;72(2):422-427.
3. Mahmood K, Wahidi MM. Straightening out chest tubes: what size, what type, and when. Clin Chest Med. 2013;34(1):63-71.
4. Rahman NM, Maskell NA, Davies CW, et al. The relationship between chest tube size and clinical outcome in pleural infection. Chest. 2010;137(3):536-543.
5. Fysh ET, Smith NA, Lee YC. Optimal chest drain size: the rise of the small-bore catheter. Semin Respir Crit Care Med. 2010;31(6):760-768.
 Prof. Langeron invited Cook Medical to his office in Paris, France, to discuss recent advancements in tracheal extubation.
Prof. Langeron invited Cook Medical to his office in Paris, France, to discuss recent advancements in tracheal extubation.
Prof. Langeron is the head of the Anaesthesia and Intensive Care Unit at the University Hospital Henri Mondor in Paris, France. He is a member of the French Society of Anesthesia & Intensive Care Medicine (SFAR) committee and a veteran in difficult airway management, and he has been involved in more than 300 publications in both national and international outlets.
Prof. Langeron is an advocate of performing a safe tracheal extubation. He said he believes a safe tracheal extubation procedure can be performed with the right team, the right medical device, and a customised strategy tailored specifically to each patient’s needs.
He also raises awareness about the importance of tracheal extubation procedures, the need to guarantee patient comfort, and the desire to avoid any risk connected to airway failure.
In the following videos, Prof. Langeron discusses post-procedure assistance with tracheal extubation, mortality, morbidity data, and the indications established in the procedural guidelines.
What is the morbidity and mortality rate for tracheal extubation, and what factors affect this?
Tracheal extubation is the third-most serious complication after tracheal intubation failure and pulmonary aspiration in anaesthesia and ICU departments, says Prof. Langeron.
6178424779001
brightcove
true
What are the guidelines for tracheal extubation?
Prof. Langeron provides an overview of the main guidelines and protocols for performing extubation procedures.
6178425333001
brightcove
true
How would you prepare for a tracheal extubation?
Prof. Langeron describes the preparation involved in an extubation procedure in accordance with the guidelines provided by the SFAR.
6178421777001
brightcove
true
What advice do you have for a physician implementing an extubation strategy?
Each physician must incorporate the specific needs of each patient into their strategy, says Prof. Langeron.
6178425334001
brightcove
true
What are your device options for performing an extubation procedure?
Prof. Langeron introduces the Cook Staged Extubation Set.
6178421387001
brightcove
true
When should the Cook Staged Extubation Set be used? How well do patients tolerate the wire guide?
Prof. Langeron explains which scenarios he thinks are most suited for the Cook Staged Extubation Set.
6178421388001
brightcove
true
Are there any cost-saving benefits to performing staged extubation procedures?
Prof. Langeron talks about his approach to following the ‘risk-benefit ratio’, a process that compares the risk factors, cost, and benefits when determining whether or not to use a device on a critically ill patient.
6178424780001
brightcove
true
Prof. Langeron is a paid consultant of Cook Medical.
Lung ultrasound is a necessary, key component in both pulmonary and critical care settings due to its high diagnostic accuracy and physicians’ ability to perform it at the bedside. In cases of pleural disease, lung ultrasound could be an essential component of care, from the initial diagnosis through clinical management and treatment.
Last year, Cook Medical had two physicians with expertise in the use of thoracic ultrasound for pleural disease share recent advances of this technique at two major industry conferences: the European Respiratory Society (ERS) International Congress and the CHEST Annual Meeting.
ERS International Congress
Luigi Vetrugno, MD, is a Professor of Anaesthesia and Intensive Care at the University Hospital of Udine in Udine, Italy. In his presentation, Thoracic ultrasound for pleural effusion, he discussed several studies he has published in recent years about the important role ultrasound plays in diagnosing and treating pleural effusion.
‘Ultrasound plays a significant role in the education of physicians’, Prof. Vetrugno said. ‘They will need to be trained to view this technology as an extension of their senses, just as many generations have viewed the stethoscope in a similar way.’
In one study co-authored by Prof. Vetrugno and published in Critical Care Medicine, thoracic ultrasound is said to not only help physicians visualise pleural effusion, but also to help them distinguish between the different types that can be present.1
Additionally, ‘TUS [thoracic ultrasound] is essential during thoracentesis and chest tube drainage as it increases safety and decreases life-threatening complications. It is crucial not only during needle or tube drainage insertion, but also to monitor the volume of the drained PLEFF [pleural effusion].’1
An observational study co-authored by Prof. Vetrugno assessed ‘the prevalence of complications related to ultrasound-guided percutaneous small-bore pleural drain insertion.’2
He stated that ‘small-bore pleural drainage device insertion has become a first-line therapy for the treatment of pleural effusions.’ In this study, ultrasound was used to assess the safety and complication rates in patients with pleural effusions. The study’s authors found ultrasound-guided placement to be a ‘safe procedure’, however, in the future, estimating the amount of pleural effusion by ultrasound will be necessary to standardise the procedure. The authors also concluded that for resident physicians ‘training and proficiency assessment should be formalised.’2
An additional article by Prof. Vetrugno in favour of ultrasound guidance can be found in Critical Ultrasound Journal, Respiratory and Pulmonary Medicine, and Annals of Intensive Care.
 He has also submitted letters to the editor regarding the importance of patient position during ultrasound procedures.
He has also submitted letters to the editor regarding the importance of patient position during ultrasound procedures.
In one letter, Prof. Vetrugno advised for the patient to remain in supine position with a mild torso elevation of 15 degrees, not a semi-recumbent position with the torso at 40-45 degrees as previous authors stated.3 ‘This means that as fluid follow the law of gravity, an overestimation of the maximal distance between partial and visceral pleura could be obtained’, he said. This theory ‘overestimates in tall males with large thoracic circumference small effusions under 200 mL and in large ones above 1000 mL.’3
According to Prof. Vetrugno, this equation allows for a high mean prediction error, however, it is recognised that ‘an urgent standardization of the method to assess PLEFF [pleural effusion fluid] with lung ultrasound is needed to reach a definite conclusion.’3
In another letter to the editor, Prof. Vetrugno echoed his concerns that larger and more standardised clinical studies should be performed before a definite conclusion is reached.4
CHEST Annual Meeting
Seth Koenig, MD, FCCP, is the Director of Education and Professor of Medicine in the Division of Pulmonary Medicine at Montefiore Medical Center in New York City, New York. In his presentation, Ultrasonography for the diagnosis and management of pleural disease, he presented about the extensive benefits of using ultrasound, most notably regarding patient safety.
Dr Koenig started his presentation with an important question, ‘How do we maximise the things we can do for our patients safely without using another service? And how does ultrasound help?’
To this question, the majority of the physician audience said that they routinely use ultrasound for general purposes, however, Dr Koenig urged them to consider ultrasound for pleural effusions. ‘For me, I use ultrasound to do everything’, he said.
 Dr Koenig explained that the use of ultrasound prevents a concept commonly known as ‘clinical and time dissociation.’
Dr Koenig explained that the use of ultrasound prevents a concept commonly known as ‘clinical and time dissociation.’
‘When you ask for another service, you invoke the ancient art of clinical and time dissociation’, he said. ‘For example, if you ask for a CAT scan, you will most likely have to involve a surgeon, an infectious disease doctor, and a pulmonologist. This can be confusing for the patient. Someone else reads the exam, you get the results of that exam, but they’re not intimately involved in the patient. This takes time for the exams to come in, plus, you have to move the patient. Is this what you do, or should we figure out a better way?’
Ultrasound, according to Dr Koenig, not only provides added clarity in terms of a diagnostic approach, but it may also reduce the number of specialists involved in the diagnostic process.
‘Every single patient that we see from the pulmonary and from critical care departments gets an ultrasound’, he said.
Additional advantages of ultrasound, according to Dr Koenig, include:
- Short learning curve
- Portable; the patient does not need to be transferred
- Decrease the need for chest x-rays
- Vessels are visible; helps to avoid post-procedural bleed
- Can reduce likelihood of a visceral laceration, vascular injury, or pneumothorax
- Ability to monitor and record complications
Dr Koenig also said that using ultrasound alleviates the need to precisely place a chest tube in the ‘triangle of safety’, a small, preferred site of insertion as determined by the British Thoracic Society.5
‘The triangle isn’t a problem anymore’, he said. ‘As long you have a nice space, you can stick the needle wherever is convenient for you and the patient because you can see everything. As long as you know where you are and your wire guide is well placed, it doesn’t matter what you insert after. The point is, if you can see it, you can do something about it.’
Dr Koenig ended his lecture by emphasising the most important reason physicians should consider the use of ultrasound for pleural diseases: the patients.
‘We have to think about our patients before we do things’, he said. ‘In 2019, I believe patients see too many doctors. They get a doctor for this, they get a doctor for that, what happens is, patients get really confused, and so if you can decrease the number of people and the number of procedures that a person has, they thank you for it, believe it or not. In conclusion, ultrasound is good for the patient, but it’s also good for you. Ultrasound helps with diagnostic and therapeutic planning. It helps to diagnose complications. It’s helpful to follow the progress of a pleural effusion, and it’s obviously extremely good for documenting post-procedure pneumothoraces.’

Prof. Vetrugno is not a paid consultant of Cook Medical.
Dr Koenig is a paid consultant of Cook Medical.
1. Brogi E, Gargani L, Bignami E, et al. Thoracic ultrasound for pleural effusion in the intensive care unit: a narrative review from diagnosis to treatment. Crit Care. 2017;21:325.
2. Vetrugno L, Guadagnin GM, Barbariol F, et al. Assessment of pleural effusion and small pleural drain insertion by resident doctors in an intensive care unit: an observational study. Clin Med Insights Circ Respir Pulm Med. 2019;13: 1179548419871527.
3. Vetrugno L, Bove T. Lung ultrasound estimation of pleural effusion fluid and the importance of patient position. Ann Intensive Care. 2018;8:125.
4. Vetrugno L, Brogi E, Barbariol F. “A message in the bottle.” Anesthesiology. 2018;128(3):677.
5. Griffiths JR, Roberts N. Do junior doctors know where to insert chest drains safetly? Postgrad Med J. 2005;81:456-458.

A pleural effusion can develop from more than 60 different causes¹ and affects 1.5 million patients per year in the US.2 A patient with a pleural disease can receive treatment from practitioners of any number of hospital specialties, including internal medicine, critical care medicine, oncology, thoracic surgery, pulmonology, radiology, cytology and histology, and laboratory sciences.
On 7 May, two visiting physicians spoke to a full training room of Cook Medical employees from many different areas, including product management, engineering, clinical affairs, regulatory affairs, marketing, and sales, on the impact of this seemingly ubiquitous condition.
Wes Shepherd, MD, and Nick Maskell, MD, visited Cook to share their experiences with and predictions about advancements in the treatment of pleural disease.
This event, titled ‘Out of breath: The devastation of pleural disease’, is part of a programme within the MedSurg division called ‘MedSurg Presents’ that allows each of the six specialties within the division to educate one another on their specific customers, products, and procedures. The goal of this programme is to allow members of each of Cook’s six clinical specialties to educate one another on their specific customers, products, and procedures. Educational opportunities like these increase the collective knowledge of the MedSurg division and allow for possible, future collaboration efforts across specialties.
Dr Shepherd was the first to present. He is currently the director of interventional pulmonology, professor of pulmonary disease and critical care medicine, internal medicine, and has an appointment as professor in the department of surgery, at the Virginia Commonwealth University School of Medicine.
‘The treatment of pulmonary disease is a relatively unfamiliar concept’, began Dr Shepherd. ‘Malignant airway disease and lung cancer were really the driving force for the advancement of interventional pulmonology. Currently, IP fellowships across the US are growing, which is allowing the methods of treatment for pulmonary disease to continuously grow and develop’.
Pleural disease can include pleural effusions, both malignant and benign, empyema, pneumothorax, and pleural tumors. Minimally invasive, non-surgical procedures to treat pleural disease can include medical thoracoscopy or pleuroscopy, thoracentesis and chest tube placement, pleural manometry, and tunnelled pleural catheters, according to Dr Shepherd. These procedures are often paired with other methods of treatment, including percutaneous dilational tracheostomy, endobronchial lung volume reduction, and endobronchial ultrasound procedures, and can be performed in different departments of the hospital, including radiology, emergency medicine, general surgery, and thoracic surgery.
Recently, more patients are receiving treatment for pleural disease at outpatient centers rather than hospitals.
‘The number of thoracentesis procedures has been steadily decreasing’, he said. ‘Because the involvement from the radiology department has been increasing, there’s been a 52% increase to outpatient hospital treatments from inpatient hospital ones’.3
Dr Shepherd also attributed the increase in outpatient procedures to the advancement of other patient treatment options, including pleuroscopy and tunnelled pleural catheters.
‘Pleuroscopy can be performed in an ambulatory setting and only requires local sedation and one or two small ports to be made’, he said. ‘By comparison, video-assisted thoracic surgery requires general anaesthesia, multiple ports to be made, and often requires an operating room. Pleuroscopy is an excellent tool for the diagnosis of the exudative effusion of unclear aetiology, and in my experience, has been superior to blind pleural biopsy.
‘Tunnelled pleural catheters have been increasingly used to treat cancer-related pleural effusions, especially when patients have recurrent effusions’, Dr Shepherd said. ‘This can be done through outpatient management and is similar to a tunnelled IV catheter. Because of the increased popularity, tunnelled pleural catheters have growing indications for benign disease, including congestive heart failure, liver disease, and kidney disease’.
Because most cancers can metastasise to the pleural space,1 Dr Shepherd also emphasized rising global smoking rates in his presentation.
‘There are over 1.3 million deaths per year worldwide due to tobacco disease, and the worldwide smoker count is supposed to increase to 1.7 billion by 2025’, he said. ‘The worldwide tobacco-related mortality rate is predicted to be 10 million deaths per year by 2030, and it’s currently at 4.8 million. Additionally, 30% of people with lung cancer worldwide will develop pleural effusions, and around 5 to 6 million of these cases will result in pleural disease from lung cancer’.
Pleural effusion is the most common pleural disease.2 Due to the prevalence of the disease, there are a variety of cross-specialty management methods, according to Dr Shepherd. These methods include thoracentesis, tunnelled pleural catheters, pleuroscopy, video-assisted thoracic surgery, chemotherapy, radiation, palliative narcotics, pleuroperitoneal shunts, tube thoracostomy, and others. Because of the widespread nature of both the origins and treatment of pleural effusion, a cross-specialty approach must be taken.
‘Recently, there has been substantial progress, evolving literature, and increasing interest in combined techniques to approaching pleural effusions’, he said. ‘However, ultimately, we’re not searching for one “holy grail” treatment method; each case must be an individualised approach. This approach should be part of a shared decision-making process across specialties’.
Dr Maskell was the second to present. He is currently a professor of respiratory medicine at the University of Bristol and an honorary consultant at North Bristol National Health Service (NHS) Trust in Bristol, UK. Dr. Maskell also cochaired the development of the 2018 British Thoracic Society (BTS) mesothelioma guidelines and is one of the chairs for the forthcoming BTS pleural disease guidelines.
‘Recently, the goals of patient care for pleural disease have started to focus more on expeditious and efficient diagnosis, as well as minimally invasive patient treatment options’, he said. ‘This is partially because many European and US centers now have ambulatory pleural services. We’re moving more and more towards patient-centered care as well; it’s more about how the patients feel and their quality of life after receiving treatment, not what their chest x-ray looks like’.
Echoing Dr Shepherd, Dr Maskell highlighted the increasing importance and adoption of tunnelled pleural catheters for the treatment of pleural disease. He highlighted the first randomised controlled trial of talc pleurodesis that was administered via a tunnelled pleural catheter as an outpatient procedure.4
‘This first randomised controlled trial showed no difference in patient-centered outcomes when comparing an indwelling pleural catheter [IPC] and talc slurry’, he said. ‘Administering talc via an IPC should become the new standard of care in patients for whom it is an appropriate method of treatment. If you have a patient in whom you want to insert an IPC, administering talc, in my experience, will likely at least double their chances of achieving pleurodesis. There is also a suggestion in the literature of potential improvement of the patient’s quality of life through this method. IPCs will become increasingly important in the future as we think of more medications and therapies that can be delivered into the pleural space’. Dr Maskell suggested cancer treatment and fluid monitoring as future uses of IPCs.
Dr Maskell also spoke about the recent rise in malignant pleural effusion cases, a condition Dr. Shepherd highlighted as a driving force in developing the field of interventional pulmonology. He related the treatment of pleural effusions back to the rising trend of patient-centred care.
‘Malignant pleural effusions are increasing every year’, he said. ‘Patient choice should continue to lead the selection of treatment for malignant pleural effusions. In my experience, outpatient talc pleurodesis is very well tolerated and increases pleurodesis rates further than daily drainage alone. Currently, regarding patients with pleural disease, I believe that only patients with pleural infections requiring chest tube drainage need treatment in an inpatient setting’.
To close, Dr Maskell spoke again about the rise of outpatient services becoming the patient-preferred standard of care to manage pleural disease.
‘People don’t want to be stuck in the hospital’, he said. ‘They want to be as mobile as possible. That’s also ideal because it can decrease the chances of additional hospital complications. Currently, 90% of my patients are now completely treated in an outpatient setting. I truly think we’re moving more towards ambulatory methods of treating patients, including telemedicine. Though it can be said that it’s more difficult to manage patients in an outpatient setting, we as care providers must learn to adapt to changing patient preferences and provide the healthcare patients want’.
Drs Shepherd and Maskell are paid consultants of Cook Medical.
1. Hooper CE, Lee YC, Maskell NA. Setting up a specialist pleural disease service. Respirology. 2010;15(7):1028-1036.
2. Krishna R, Rudrappa M. Pleural effusion. StatPearls Web site. https://www.ncbi.nlm.nih.gov/books/NBK448189/. Updated February 11, 2019. Accessed August 26, 2019.
3. Duszak R, Chatterjee A, Schneider D. National fluid shifts: fifteen-year trends in paracentesis and thoracentesis procedures. J Am Coll Radiol. 2010;7(11):859-864.
4. American Thoracic Society. Management of malignant pleural effusions. Am J Respir Crit Care Med. 2000;162(5):1987-2001.







