This blog content derives from the January 2016 EVT Europe Supplement: Global Approaches To Type B Dissection article written by Michael D. Dake, MD.
During the last 5 to 10 years, we have witnessed an increasingly sharp focus on many aspects of aortic dissection. This concentration is not solely directed at treatment strategies; rather, it has produced important new insights into diagnosis, imaging, classification, prognostic features of disease progression, and follow-up regimens.
The impetus for this heightened understanding is fueled by a broader range of interested specialists and is based on the emergence of endovascular procedures, including thoracic endovascular aortic repair (TEVAR), that provide less invasive alternatives to open surgical repair to address manifestations of the disease.
Around the world, TEVAR is now acknowledged as the treatment of choice for acute, complicated type B aortic dissection. Traditionally, this includes a type B dissection associated with rupture, symptomatic branch vessel involvement, persistent pain, or difficult-to-control hypertension.
DISEASE PROGRESSION
Recently, attention has been directed to the risk of disease progression within the first 3 to 5 years after diagnosis of a type B dissection initially considered uncomplicated. There is now awareness of a variety of disease features observed at the time of diagnosis that appear to represent risk factors for subsequent disease progression.
The majority of these high-risk features are anatomically based, often related to aortic dimensions measured on imaging studies. In the future, it is likely that additional prognostic factors from physiological, hemodynamic, or aortic wall biological studies will be recognized and will contribute to additional understanding of which patients with initially uncomplicated dissection may be at increased risk of early disease progression, including rupture or aneurysm formation.
In the meantime, one of the frequent topics of discussion and debate currently featured at cardiovascular meetings and in articles that provide a perspective on current management of type B dissection is whether we should offer TEVAR treatment to patients who have an initially uncomplicated process but harbor multiple high-risk features for progression. And, if so, when should treatment occur?
Obviously, immediate TEVAR will be performed on patients who present with a life-threatening complication (rupture or branch vessel ischemia) at the time of diagnosis, if anatomically suitable and feasible. Current controversies focus on whether it’s possible to identify a subgroup of patients who are initially deemed uncomplicated, but who would benefit from an essentially prophylactic TEVAR procedure to potentially prevent subsequent complications, which may or may not present emergently.
If we examine what we know in the current snapshot in time, the answer to this question is unclear, but there exists an abundance of opinions. Given the best available data, how can we begin to analyze the risks and benefits for such a strategy?
WHAT DO WE KNOW?
First, in patients managed with what is currently the best medical therapy, the risk of death within the first 30 days after diagnosis of acute type B dissection is approximately 10% to 11%.1,2 We can assume that an overwhelming majority of these patients were deemed initially uncomplicated; otherwise, endovascular or open surgical interventions would have been performed to manage any complications. The majority of the early deaths in this group are due to aortic rupture that occurs within the initial 14 days after diagnosis. Could this early mortality rate be improved by early TEVAR therapy in a subgroup of patients with a high-risk profile based on some composite of features that can predict disease progression or early complications? Currently, we don’t know.
What we do know is that early treatment of type B aortic dissection within the first 48 to 72 hours, or even within a week, is associated with an increased risk of retrograde type A dissection—at least when TEVAR is used to manage patients with an acute complicated process (Figure 1). This dreaded catastrophe, which is not universally fatal and not exclusively due to the endoprosthesis, may occur at a rate as high as 3% to 4%.3-5 Based on published meta-analyses, approximately 23% of these cases were diagnosed during the procedure (8%) or immediately periprocedurally (15%), with associated mortality rates of 70% and 50%, respectively.3 This is compared to an estimated mortality rate of 30% for cases of retrograde type A dissection diagnosed after hospital discharge.
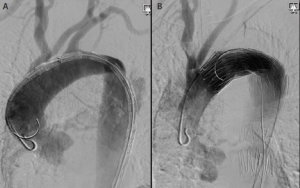
Figure 1. A 68-year-old woman with an acute type B aortic dissection. The left anterior oblique aortogram with an endograft in the aortic arch just beyond the left carotid artery origin prior to deployment. The ascending aorta is normal with a guidewire and flush catheter against the outer anterior wall (A). Aortogram postdeployment of the endograft with a flush catheter within the true lumen of the ascending aorta displaced away from the anterior wall by a false lumen caused by retrograde type A aortic dissection. The patient went to the operating room for open repair of the ascending segment and recovered uneventfully (B).
With this risk in mind, what other signposts can we look to in order to direct our future management strategies? The much-publicized results of the INSTEAD trial raised awareness of the frequency of late aortic-related events in patients with type B dissection deemed initially uncomplicated who were treated more than 14 days after the onset of symptoms (median of approximately 8 weeks).6 The so-called INSTEAD-XL extension of the original protocol provided follow-up results between 2 and 5 years after the initial randomization of treatment to endograft placement plus medical therapy (n = 72) or medical therapy alone (n = 68).7
The landmark analysis of the outcomes from 2 to 5 years in the two groups detailed 15 deaths in the optimal medical therapy arm over this time period and none in the TEVAR-plus-medical-therapy group. Of the 15 deaths in the optimal medical therapy arm, all but two were due to a known aortic rupture or sudden death (defined as a death within 1 hour in patients with known absence of coronary or structural heart disease).
During the course of the 5-year study, 26% of the medical therapy patients underwent crossover to TEVAR placement (14 cases, including five emergencies) or conversion to open repair (four cases), both for enlarging false lumen diameters. Conversely, in the TEVAR group, additional stent graft placement was required in seven cases and conversion to open repair in three cases, for a total reintervention rate of 13% over the same time period. Notably, there was no periprocedural mortality after crossover to TEVAR or conversion to open repair.
Over the 5-year study, the difference in all-cause mortality was not statistically significant (P = .13) between TEVAR plus optimal medical therapy (11.1%) and optimal medical therapy alone (19.3%); however, the difference in aortic-specific mortality at 5 years was statistically significant (6.9% versus 19.3%, P = .04). In terms of disease progression through 5 years, there was a 19.1% absolute risk reduction with TEVAR (27.0%) when compared to medical therapy (46.1%). This difference between the outcomes in the two groups was statistically significant (P = .04). Of note, in the TEVAR group, there was one case (1.4%) of retrograde type A dissection.
So, given these data, what can we make of the opportunity for reducing the mortality rates and disease progression by early TEVAR intervention in patients with initially uncomplicated type B aortic dissection?
MORE QUESTIONS THAN ANSWERS
Well, the trend of podium opinions around the world indicates that if we could confidently define a group of patients with a high-risk profile for disease progression, based on various clinical and anatomic manifestations of their dissections, a strategy of early TEVAR may be warranted to prevent complications, including rupture and false lumen dilatation. A number of criteria composed of high-risk features have been shown to predict those patients who are likely to progress from initially uncomplicated to a complicated type B status within the early- to mid-period after diagnosis.
Unfortunately, no one criterion or composite of features has been consensually agreed upon or proven to precisely define such a group and their specific risks of complications, or to predict within what time frame after diagnosis they are most susceptible. Consequently, we proceed much like a jury weighing each proposed high-risk feature until we accumulate a preponderance of evidence that meets a threshold and triggers consideration of TEVAR in a patient with an initially uncomplicated disease process.
Therefore, the question at present is whether the procedural risk of TEVAR is outweighed by the benefit of a prophylactic or preventive therapy applied to a group of patients that is yet to be strictly defined. Suffice it to say, no one knows for sure.
Another unknown that could influence our decision making if better understood, is the appearance of the aorta on the most recent imaging surveillance in patients who experienced rupture or a sudden death in the midterm (2 to 5 years) after diagnosis. Regrettably, this particular imaging follow-up was not available from the INSTEAD-XL data. There were 13 cases of rupture or sudden death in the optimal medical therapy group in INSTEAD-XL between 2 and 5 years, but we did not know whether these patients had progressive false lumen dilatation on successive surveillance imaging exams, and if so, to what degree.
The lack of these results from a well-controlled, prospective clinical trial highlights the difficulty of strict monitoring for possible disease progression at intervals frequent enough to identify patients at high risk for catastrophic or impending complications. Clearly, this is even more challenging in a real-world setting where patient compliance with prescribed follow-up protocols, including CT imaging, is even more difficult to achieve.
The bottom line is that we just don’t know if a patient at risk for catastrophic events is following a personal trajectory of disease progression that reaches a threshold, such as a cutoff in the aneurysm diameter, that can predict a high risk of mortality. By tracking with vigilant imaging surveillance, we could potentially avoid rupture by crossing over to TEVAR at a time that minimizes procedural risks. Also, if a monitoring strategy to securely minimize late aortic-specific mortality is possible, at what point of follow-up would we lose the ability to achieve the same desirable aortic remodeling observed with TEVAR in the more acute setting?
Clearly, we now have many more questions than answers.
GOLDILOCKS DILEMMA
For patients who have been traditionally classified as having acute uncomplicated type B aortic dissection, we are slowly growing comfortable with a consensus view that their conditions are actually only initially uncomplicated. Rather, they exhibit certain anatomic and clinical features that predict a high risk for disease progression and aortic-related events sometime within 60 months after diagnosis.
In this group of patients, especially those who may not comply with prescribed follow-up protocols, a more aggressive treatment approach incorporating early TEVAR, if anatomically suitable, may be considered. The timing of such a procedure may need to be individualized based upon patient factors. In order to minimize the risk of retrograde type A dissection associated with the procedure, acute TEVAR may not be advisable. Rather, a strategy of delayed TEVAR performed from 1 week to 3 months after diagnosis has been advocated by some authorities.
This approach acknowledges our current understanding of the evolving spectrum of the acute type B aortic dissection and the importance of stratification of management strategies based on certain anatomic and clinical features of the disease. So, in the end, today, we find ourselves facing the proverbial Goldilocks dilemma in terms of deciding the optimal time to intervene with TEVAR.
We don’t want to intervene with TEVAR too soon in the acute phase when the risk of fatal type A retrograde dissection may be the highest, but we don’t want to wait too long and lose the opportunity to prevent a catastrophic rupture in poorly compliant patients who become lost to follow-up, or the chance to optimally remodel the aorta post-TEVAR when disease progression is too advanced or too chronic. We want to mitigate all these risks and perform TEVAR at just the right time.
CONCLUSION
The dilemma is all too real, and identifying the right time to intervene is currently an unmet challenge. Successfully defining the risks/benefits regarding the timing of TEVAR will undoubtedly contribute greatly to improved outcomes for our patients with acute, initially uncomplicated type B dissection.
Michael D. Dake, MD, is the Thelma and Henry Doelger Professor in the Department of Cardiothoracic Surgery, Stanford University School of Medicine, and Medical Director, Catheterization and Angiography Laboratories, Stanford University Hospital in Stanford, California.
Get stories like these sent to your email.
Sign up for our quarterly email newsletter to receive physician stories, product news, training opportunities and more in your inbox.
Dr. Dake is a paid consultant of Cook Medical.
-
Hagan PG, Nienaber CA, Isselbacher EM, et al. The International Registry of Acute Aortic Dissection (IRAD): new insights into an old disease. JAMA. 2000;283:897-903.
-
Estrera AL, Miller CC, Safi HJ, et al. Outcomes of medical management of acute type B aortic dissection. Circulation. 2006;114(1 suppl):S384-S389.
-
Eggebrecht H, Thompson M, Rousseau H, et al. Retrograde ascending aortic dissection during or after thoracic aortic stent graft placement: insight from the European Registry on Endovascular Aortic Repair Complications. Circulation. 2009;120(1 suppl):S276-S281.
-
Khoynezhad A, White RA. Pathogenesis and management of retrograde type A aortic dissection after thoracic endovascular repair. Ann Vasc Surg. 2013;27:1201-1206.
-
Williams JB, Andersen ND, Bhattacharya SD, et al. Retrograde ascending aortic dissection as an early complication of thoracic endovascular aortic repair. J Vasc Surg. 2012;55:1255-1262.
-
Nienaber CA, Kische S, Akin I, et al. Strategies for subacute/chronic type B aortic dissection: the investigation of stent grafts in patients with type B aortic dissection (INSTEAD) trial 1-year outcome. J Thorac Cardiovasc Surg. 2010;140(6 suppl):S101-S108.
-
Nienaber CA, Kische S, Rousseau H, et al. Endovascular repair of type B aortic dissection: long-term results of the randomized investigation of stent grafts in aortic dissection trial. Circ Cardiovasc Interv. 2013;6:406-416.
